Considerations for Assessment of Reproductive and Developmental Toxicity of Oligonucleotide-Based Therapeutics | Nucleic Acid Therapeutics

Event: HESI DART Committee Thyroid Hormone Assessment Workshop - HESI - Health and Environmental Sciences Institute
DART-02: Prenatal Development Studies of 4-Methylcyclohexanemethanol (CASRN 34885-03-5) in Sprague Dawley (Hsd:Sprague Dawley SD
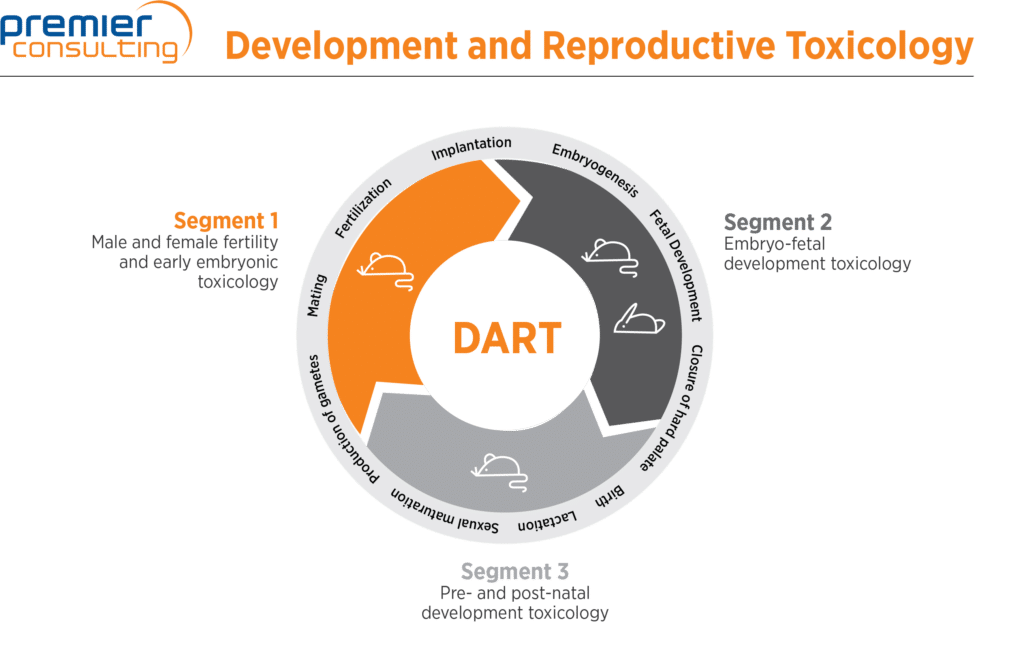
Developmental And Reproductive Toxicology (DART) Studies: How Do They Fit Into Your Program? | Premier Consulting

DART - "Developmental And Reproductive Toxicology (database / base de données)" by AcronymsAndSlang.com
Pharmaceutical toxicology: Designing studies to reduce animal use, while maximizing human translation

Developmental and Reproductive Toxicology (DART) - HESI - Health and Environmental Sciences Institute
Developmental and Reproductive Toxicology (DART) - HESI - Health and Environmental Sciences Institute
Developmental And Reproductive Toxicology (DART) Studies What Are They And How Do They Fit Into Your Program

Immunoaffinity nanogold coupled with direct analysis in real time (DART) mass spectrometry for analytical toxicology - Analytical Methods (RSC Publishing)

Characterization of bone abnormalities from micro-CT images for evaluating drug toxicity in developmental and reproductive toxicology (DART) studies | Semantic Scholar

FDA Guidelines For Developmental and Reproductive Toxicology (DART) Studies for Small Molecules | Leaders in Pharmaceutical Business Intelligence (LPBI) Group

Carcinogenicity *General Toxicology **DART **Safety Pharmacology HighMedium Low Genetic Toxicity Pharmacokinetics Pharmacology Study Group Priority Study. - ppt download