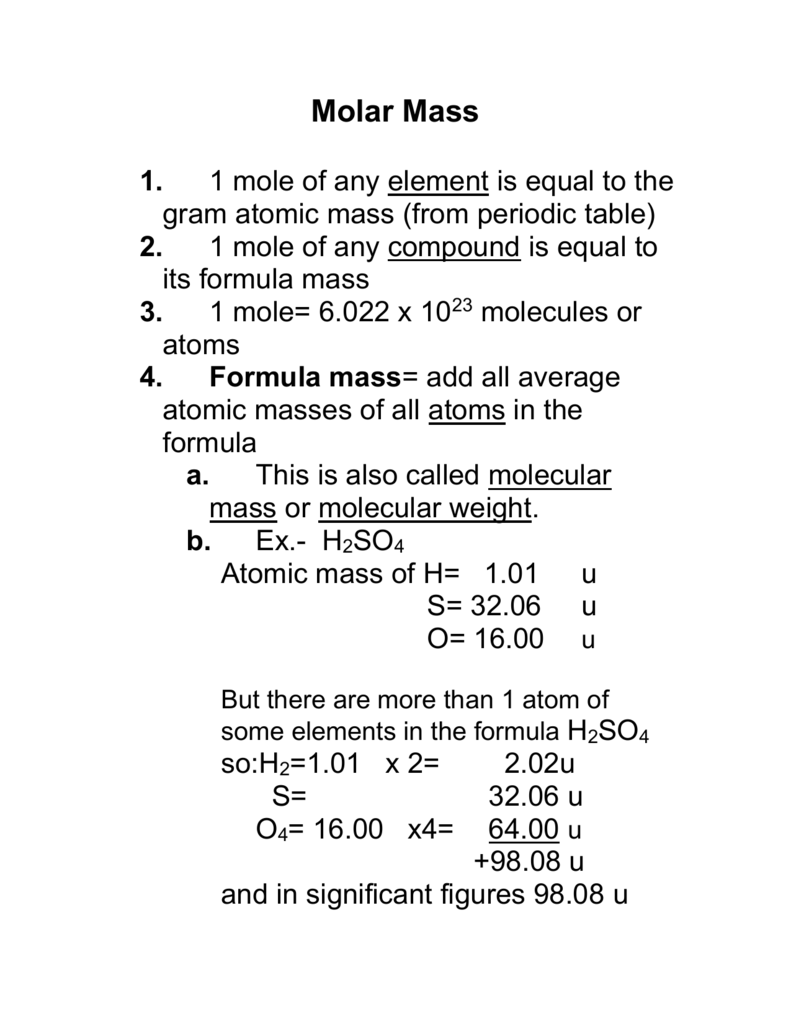
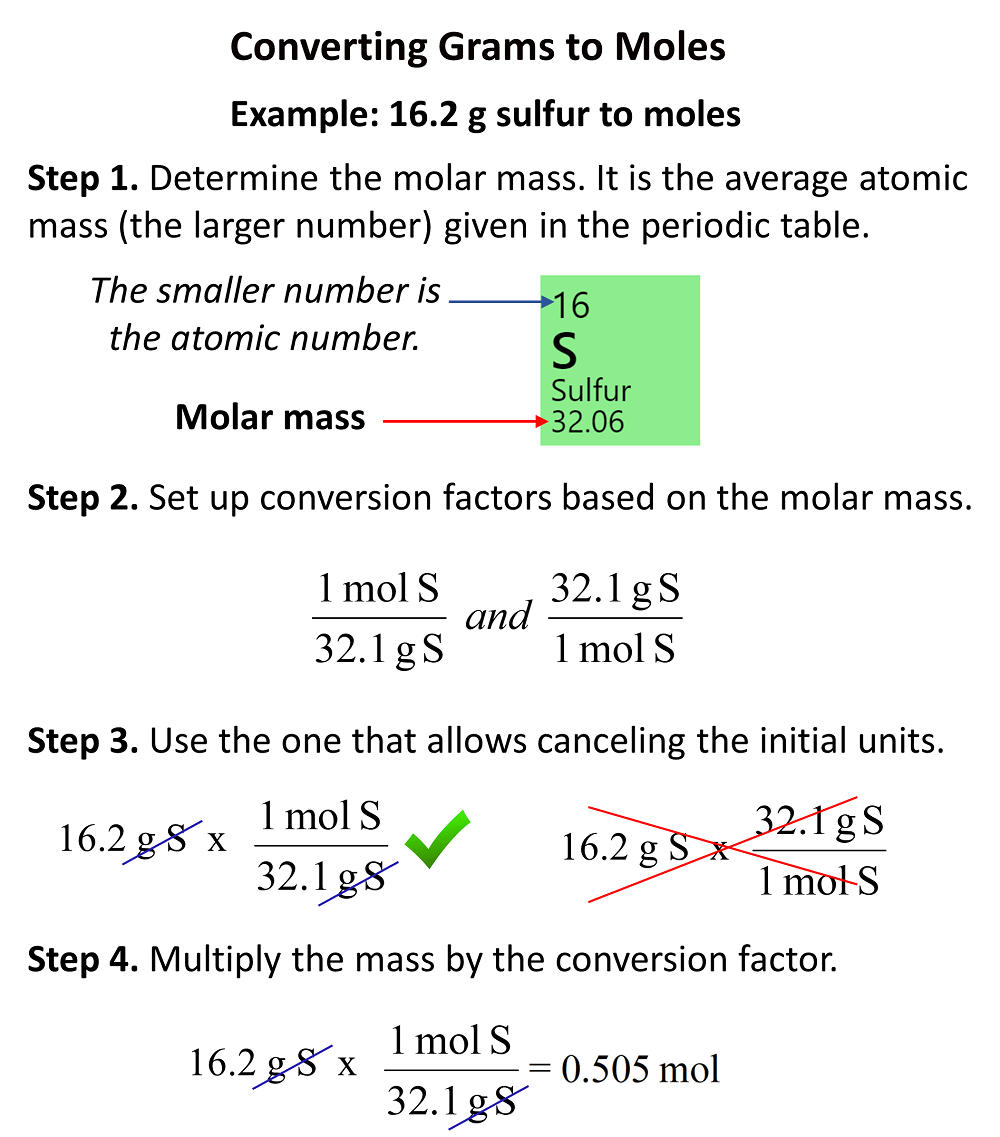
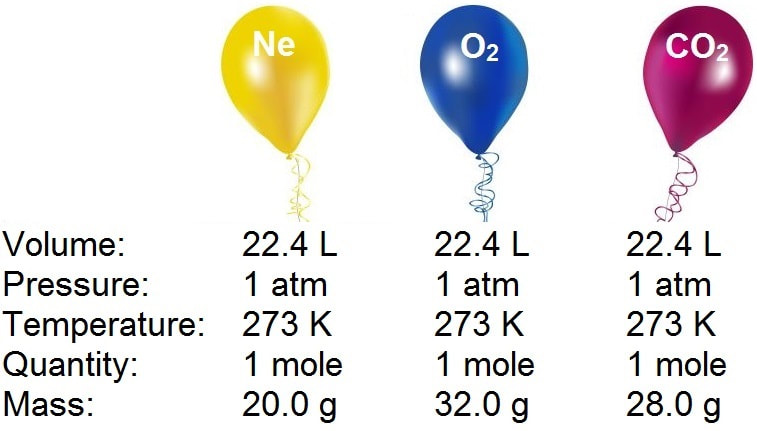
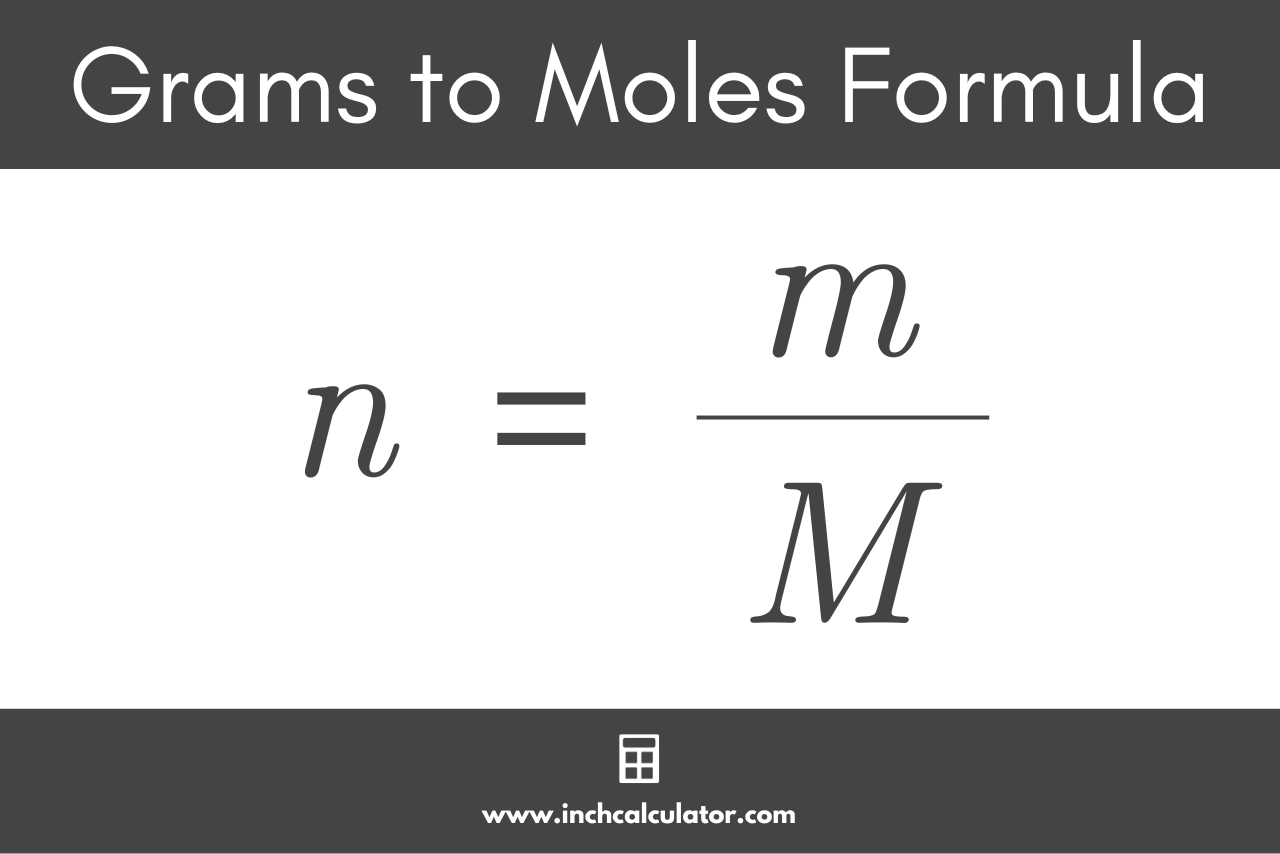Analysis of Two Definitions of the Mole That Are in Simultaneous Use, and Their Surprising Consequences | Journal of Chemical Education

Which of the following contains the same number of atoms as 4.032g of hydrogen atoms? A. 1 mole of H2 - Brainly.com

Calculate the mass and charge of one mole of electrons. Given: Mass of one electron = 9.10 × 10^-31 kgCharge of one electron = 1.602 × 10^-19 coulomb

The Mole Standards 1 dozen = 1 gross = 1 ream = 1 mole = x There are exactly 12 grams of carbon-12 in one mole of carbon ppt download

If one mole of carbon atoms weighs 12 grams, what is the mass (in grams) of 1 atom of carbon?... - YouTube

The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download